Journal club: Zheng et al, 2019
Zheng, Jian-Qing, Xiao-Yun Zhou, Celia Riga, and Guang-Zhong Yang
International Conference on Robotics and Automation (ICRA), pp. 8747-8753. IEEE, 2019.
DOI: https://doi.org/10.1109/ICRA.2019.8793918
The current standard of intra-operative navigation during Fenestrated Endovascular Aortic Repair (FEVAR) calls for the need of 3D alignments between inserted devices and aortic branches. The navigation commonly via 2D fluoroscopic images, lacks anatomical information, resulting in longer operation hours and radiation exposure.
In this paper, a skeleton instantiation framework of Abdominal Aortic Aneurysm (AAA) from a single 2D fluoroscopic image is introduced for real-time 3D robotic path planning. A graph matching method is proposed to establish the correspondences between the 3D preoperative and 2D intra-operative AAA skeletons, and then the two skeletons are registered by skeleton deformation and regularization in respect to skeleton length and smoothness. Furthermore, deep learning was used to segment 3D preoperative AAA from Computed Tomography (CT) scans to facilitate the framework automation.
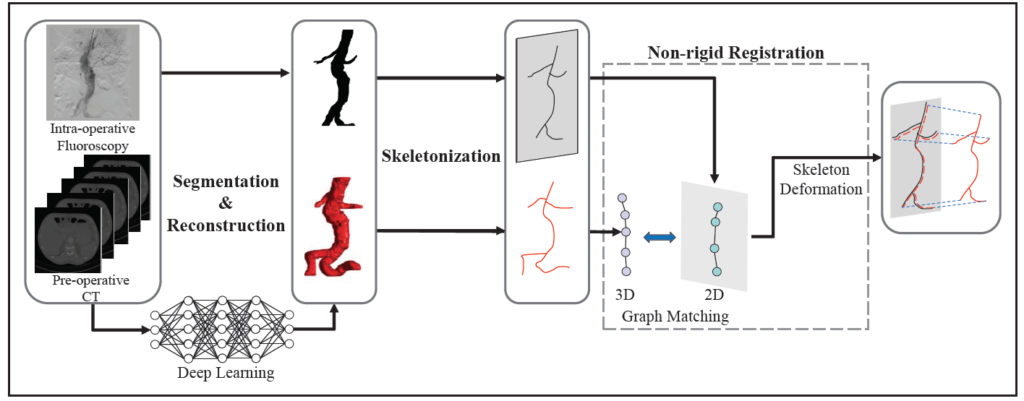
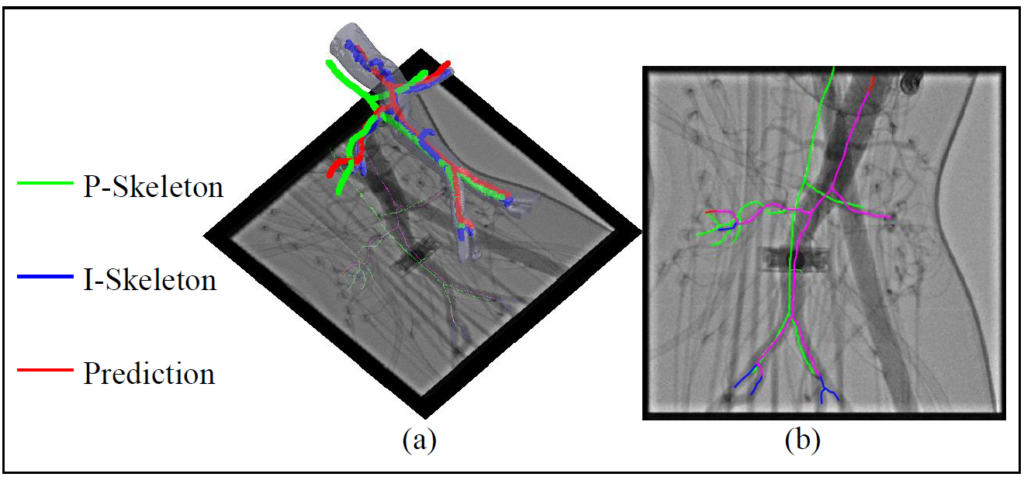
Simulation, phantom, and patient AAA data sets have been used to validate the proposed framework. In this work, the endovascular evaluator (BR Biomedicals Pvt. Ltd.) with strings was used as a phantom with intra-operative deformations. 3D distance error of 2mm was achieved in the phantom setup. Performance advantages were also achieved in terms of accuracy, robustness, and time-efficiency.
Discussion
Zheng et al. validate their method’s accuracy and time-efficiency. Nevertheless, there are some concerns regarding their proposed framework: (i) It is sensitive to topology variance using the skeleton graph matching approach. This work limits to 5 branches (aortic aneurysm, renal arteries, iliac arteries) which are required to be the longest ones. In other cases, the method performance can not be guaranteed. (ii) This work only validates the 2D accuracy of the experiment on patient data, due to the fact that it is hard to acquire the ground truth in 3D intra-op cases for validation. (iii) The proposed work is targeting accurate 3D path planning, but skeleton-based guidance is the ideal case. It needs to be close to reality respecting the capability of inserted devices (guidewires, catheters, etc).
For further improvement based on their work, some ideas are presented: (i) To match the 3D shape with the 2D shape, other features can be selected, such as the edge feature. Learning-based 2D/3D deformable registration can be applied to improve the robustness. (ii) Lacking the ground truth in 3D intra-op cases, other intra-op images (e.g., TEE, IVUS) can be referred to as ground truth for validation and optimization. (iii) Reinforcement learning for path planning of traditional guidewires/catheters could be exploited, or path planning based on skeleton could be improved respecting the steerable continuum robot kinematics.