NTA 1 – Medical device development and Translation
NTA1 took place over the course of three days from March 22 to March 24, 2021. It was co-organized by UNISTRA and UPC and took place virtually.
Day 1 focused on interdisciplinary clinical research and medical device development; Day 2 was aimed at pre-clinical and clinical development and clinical translation, and Day 3 continued the clinical translation theme.
The full program is listed below. Part of the slides is available at the end of the page.
Program
Monday 22 March 2021
9:00 – 12:00 Session 1 – Interdisciplinary clinical research
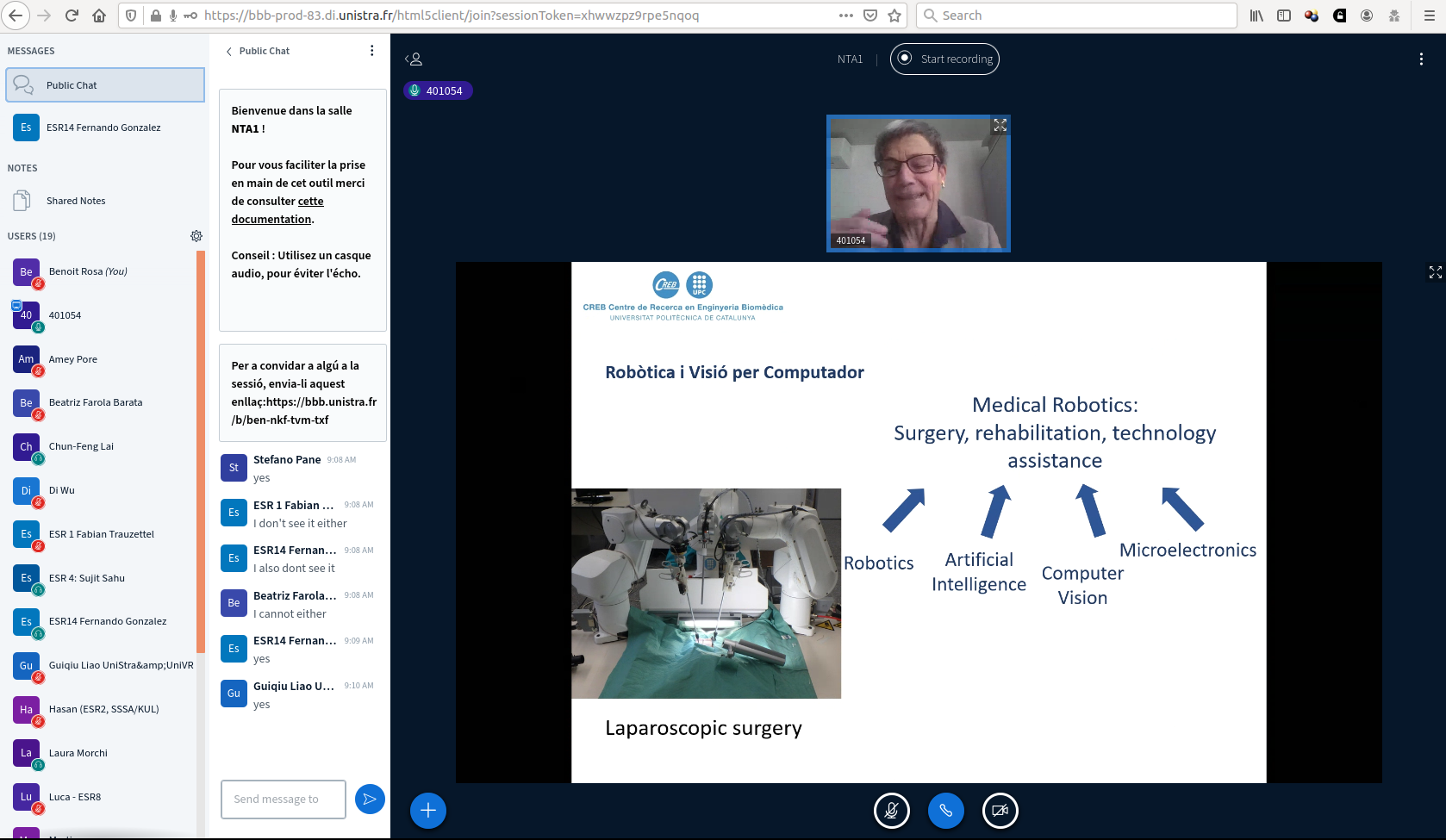
9:00 – 10:00 Creation of a company in medical robotics. From the lab to the market Alicia Casals, UPCS Barcelona
10:15 – 11:15 Translation of robotic devices – practical aspects Florent Nageotte & Philippe Zanne, Strasbourg University
11:30 – 12:00 Clinical need assessment Michalina Gora, CNRS
13:30 – 16:15 Session 2 - Medical device development
13:30 – 15:15 CE Marking European regulations Michalina Gora, CNRS
15:30 – 16:15 Good manufacturing practice Benoit Rosa, CNRS
Tuesday 23 March 2021
9:00 – 12:00 Session 3 - Pre-clinical and Clinical validation
9:00 – 10:00 Animal studies regulations Chrystelle Po, Strasbourg University
10:15 – 11:00 Clinical trial regulations Michalina Gora, CNRS
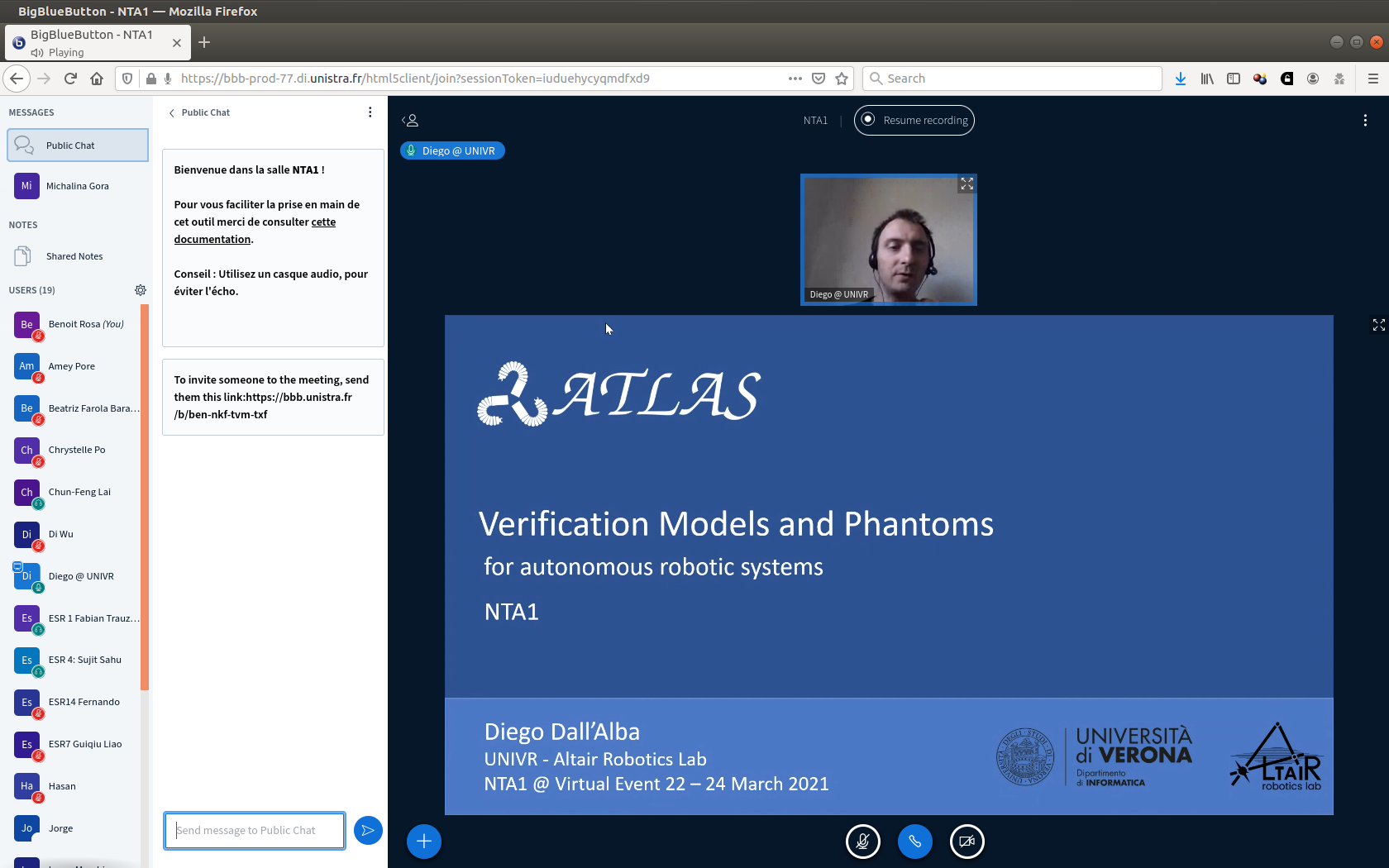
11:15 – 12:00 Verification Models and phantoms Diego Dall’Alba, Verona University
13:30 – 16:30 Session 4 - Clinical translation
13:30 – 14:00 Clinical research Nurse’s perspective on Clinical Translation Catriona Grant, MGH, Boston USA
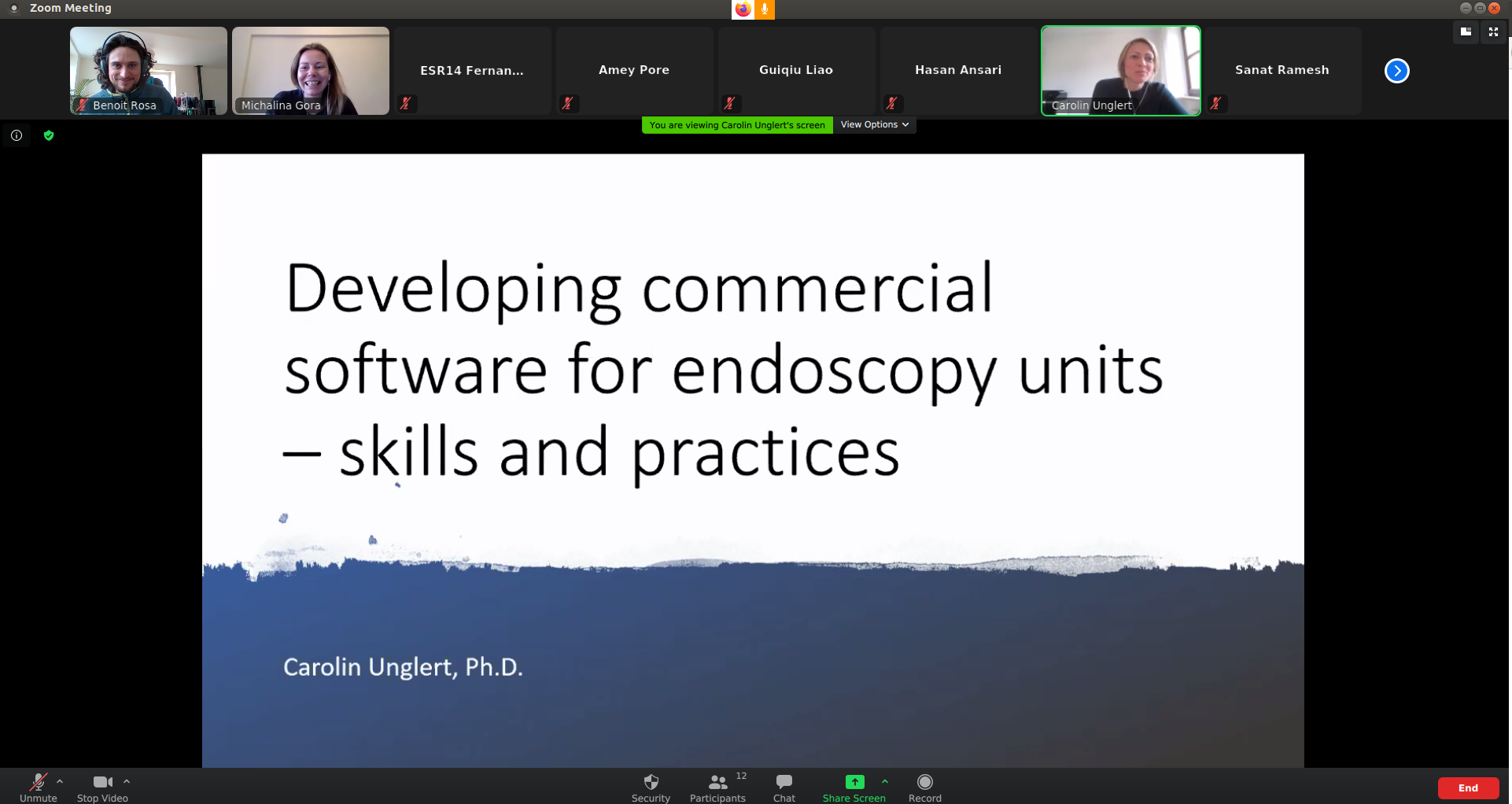
14:15 – 15:00 Developing commercial software for endoscopy units – skills and practices Carolin Unglert, GE, Munich, Germany
15:15 – 15:45 Clinical data gathering and management Benoit Rosa, CNRS
Wednesday 24 March 2021
9:00 – 10:30 Session 5 - Clinical translation
09:15 – 10:00 OCT capsule - from bench to bedside Michalina Gora, CNRS
10:00 – 10:30 Interdisciplinary communication Michalina Gora, CNRS
Available Presentations